The work entitled "BLD10/CEP135 Is a Microtubule-Associated Protein that Controls the Formation of the Flagellum Central Microtubule Pair" has just been accepted in Developmental Cell.
Cilia and flagella are involved in a variety of processes and human diseases, including ciliopathies and sterility. Their motility is often controlled by a central microtubule (MT) pair localized within the ciliary MT-based skeleton, the axoneme. We characterized the formation of the motility apparatus in detail in Drosophila spermatogenesis. We show that assembly of the central MT pair starts prior to the meiotic divisions, with nucleation of a singlet MT within the basal body of a small cilium, and that the second MT of the pair only assembles much later, upon flagella formation. BLD10/CEP135, a conserved player in centriole and flagella biogenesis, can bind and stabilize MTs and is required for the early steps of central MT pair formation. This work describes a genetically tractable system to study motile cilia formation and provides an explanation for BLD10/CEP135's role in assembling highly stable MT-based structures, such as motile axonemes and centrioles.
Reference: BLD10/CEP135 Is a Microtubule-Associated Protein that Controls the Formation of the Flagellum Central Microtubule Pair. Carvalho-Santos Z, Machado P, Alvarez-Martins I, Gouveia SM, Jana SC, Duarte P, Amado T, Branco P, Freitas MC, Silva ST, Antony C, Bandeiras TM, Bettencourt-Dias M. Dev Cell. 2012 Aug 14;23(2):412-24.
The work entitled "Structural and functional insights into a dodecameric molecular machine - The RuvBL1/RuvBL2 complex" has just been accepted in the Journal of Structural Biology.
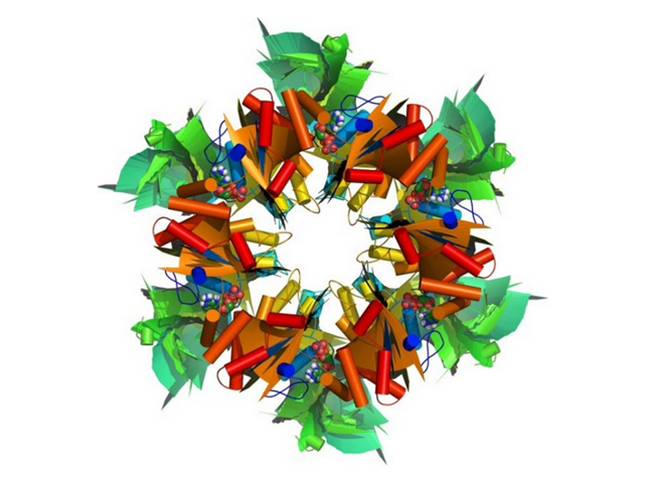
RuvBL1 (RuvB-like 1) and its homolog RuvBL2 are evolutionarily highly conserved AAA+ ATPases essential for many cellular activities. They play an important role in chromatin remodeling, transcriptional regulation and DNA damage repair. RuvBL1 and RuvBL2 are overexpressed in different types of cancer and interact with major oncogenic factors, such as β-catenin and c-myc regulating their function. We solved the first three-dimensional crystal structure of the human RuvBL complex with a truncated domain II and show that this complex is competent for helicase activity. The structure reveals a dodecamer consisting of two heterohexameric rings with alternating RuvBL1 and RuvBL2 monomers bound to ADP/ATP, that interact with each other via the retained part of domain II. The dodecameric quaternary structure of the R1ΔDII/R2ΔDII complex observed in the crystal structure was confirmed by small-angle X-ray scattering analysis.
Interestingly, truncation of domain II led to a substantial increase in ATP consumption of RuvBL1, RuvBL2 and their complex. In addition, we present evidence that DNA unwinding of the human RuvBL proteins can be auto-inhibited by domain II, which is not present in the homologous bacterial helicase RuvB. Our data give new insights into the molecular arrangement of RuvBL1 and RuvBL2 and strongly suggest that in vivo activities of these highly interesting therapeutic drug targets are regulated by cofactors inducing conformational changes via domain II in order to modulate the enzyme complex into its active state.
Reference: Gorynia S, Bandeiras TM, Pinho FG, McVey CE, Vonrhein C, Round A, Svergun DI, Donner P, Matias PM, Carrondo MA. Structural and functional insights into a dodecameric molecular machine - The RuvBL1/RuvBL2 complex. J Struct Biol . 2011 Sep 10.